Radiofrequency (RF) ablation involves delivering medium-to-high-frequency alternating current, which is electrically conducted to cardiac tissue that constitutes a part of the circuit. The unipolar circuitry contains the generator, transmission lines, catheter, cardiac tissue, interposed tissue and dispersive electrode (ground pad/indifferent electrode). Bipolar systems, whereby the RF current flows between two electrodes, either on the same catheter or on different catheters placed on opposite sides of the cardiac tissue, also exist. The frequency spectrum commonly used for RF ablation is in the range of 300–1,000 kHz because lower frequencies would stimulate the cardiac muscle and induce polymorphic arrhythmias, and higher frequencies are associated with high energy loss along the transmission lines.1
When RF current is applied, the charged ions (Na+, K+, Cl–) within the tissue attempt to follow the changes in the direction of the alternating current. These ion oscillations generate heat due to friction, also known as resistive heating. Thus, resistive heating of the tissue starts immediately when RF energy is applied but is absorbed by the first 1–1.5 mm of tissue. The remainder of heating occurs as conduction from this rim to the surrounding tissue (conductive heating), which takes time to equilibrate; therefore, a full-grown lesion may require 30–60 seconds to produce.1
RF ablation catheters have gone through many changes and innovations since their infancy. Solid-tip 4–10 mm catheters soon evolved into irrigated catheters, which permit higher power applications while protecting the tissue in contact with the catheter tip from overheating.2 Contact force (CF) sensing catheters were developed more than a decade ago and provide feedback on the quantity and direction of force the operator is applying to the tissue.2 More recently, mini- and micro-electrodes that provide higher fidelity signals and can evaluate changes in electrograms while ablating have been incorporated in the tips of ablation catheters, as have thermocouples that accurately measure tissue temperature at the catheter tip even in the context of irrigation.3–5
The importance of lesion quality markers
Complete and durable long-term pulmonary vein isolation (PVI) is the goal of every atrial fibrillation (AF) ablation procedure.6 Acute PVI is readily achieved with modern technology and is demonstrated by an entrance and exit block between the left atrium and the pulmonary vein at the completion of the procedure.7 On the other hand, long-term PVI failure is only suspected in the event of AF recurrence. From a technical standpoint, lesions need to be created uniformly and contiguously during RF applications.8 To that end, real-time lesion quality markers have become widely used, indicating to operators when to cease applying RF energy, knowing that a transmural and durable lesion is likely to have been created. Operator factors contributing to the lesion are the force that the tip of the catheter applies to the tissue, the duration of the applied energy and the RF power. The stability of the catheter tip is also essential. The spacing between the lesions is an important factor that is not related to individual lesions but rather to the set of lesions as a contiguous line and, therefore, significantly impacts the durability of the procedure. Too much movement of the catheter from the previous to the subsequent lesion can leave gaps, and too little movement leads to greater overlap; this results in delivery of more lesions, prolonging the procedure and increasing the risk of a complication.
Initial lesion quality markers relied on input variables such as CF, contact time and power. Although these factors greatly influence lesion size and volume, they do not provide feedback to the operator on the tissue response to energy delivery. This has led to the development of lesion markers based on output measures such as catheter tip temperature and impedance.4,5 These various input and output factors and metrics incorporating combinations of them are considered in this article.
Factors contributing to radiofrequency lesions
Contact force
Multiple CF catheters and systems are available, including ThermoCool SmartTouch® (Biosense Webster Inc., Irvine, CA, USA), TactiCath™ (Abbott Laboratories, Chicago, IL, USA), IntellaNav StablePoint™ (Boston Scientific, San Jose, CA, USA), AcQBlate® FORCE (Acutus Medical, Carlsbad, CA, USA) and AlCath Force (Biotronik, Berlin, Germany). The ThermoCool SmartTouch® module calculates the force that is applied to the tip of the catheter by measuring the degree of deformation of a tiny spring that internally connects the tip to the shaft.9 TactiCath™ uses light interferometry technology with three fibre-optic sensing cables and, through interference analysis, can compute both the orientation and magnitude of CF.10 Further similarities and differences between these and the other catheters are beyond the scope of this paper.
CF is a major determinant of RF lesion size.11 Furthermore, targeting 20–50 g of CF while ablating healthy atrial tissue lowers the incidence of AF recurrence and reduces the rate of cardiac perforation and tamponade.12 A randomized, multicentre UK study demonstrated that the availability of CF data was associated with reduced acute pulmonary vein reconnection, though 1-year success rates as assessed by 7-day Holter recordings at 6 and 12 months were not improved.13 The TOCCATA study showed that high CF readings were observed not only during ablation times but also during mapping (Touch+™ for Catheter Ablation [TOCCATA]; ClinicalTrials.gov Identifier: NCT01223469).14 The investigators suggested that CF catheters may increase the safety and effectiveness of RF ablation due to better control of lesion size.14
Application duration
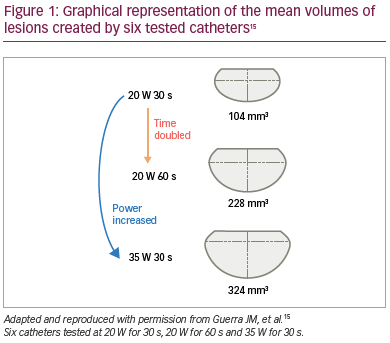
A longer duration of RF application contributes to the total energy delivered and, due to augmented conductive heating, to lesion volume. Guerra et al. undertook in vitro testing of six irrigated-tip catheters at various powers and with 30- or 60-second application durations with a consistent CF of 10 g.15 They concluded that the tip design did not significantly affect the lesion volume and that there were only marginal differences in safety. Nevertheless, duration did affect the lesion volume at both power settings, as seen in Figure 1.15
Power delivery
The power delivered is directly related to lesion size and depth. In the study described above, an increase in power from 20 W to 35 W tripled the lesion volume, as depicted in Figure 1.15 Higher power settings result in increased current flow and higher temperatures deeper in the tissue but also lead to high temperatures at the catheter tip/tissue interface, which requires increased irrigation rates to avoid excessive temperature rises that may result in steam pops or char formation.16
Lesion spacing
Inter-lesion distance (ILD) is of critical importance because, a ‘break’ in the line of a wide-area circumferential ablation (WACA) can result in the reconnection of the pulmonary veins and recurrence of AF, even if the individual lesions are transmural and durable. ILD is the centre-to-centre distance between lesion tags (and is therefore unaffected by the chosen lesion tag diameter) rather than the gap between the edges of lesion tags, which can be made larger or smaller by changing the tag size. Projected tags (flat circles on the geometry surface) can be misleading with regard to ILD because the surface projections depend heavily on how accurately the left atrium geometry has been created and edited, whereas 3D spherical lesion tags have a specific location in space.17
An ILD of less than 5.4 mm or 4.4 mm for the right anterior and posterior pulmonary veins, respectively, and 5.5 mm or 5.1 mm for the left anterior and posterior pulmonary veins, respectively, were optimal for first-pass isolation in one study, in keeping with previous work.17,18
Radiofrequency lesion quality markers
Force–time integral
Force–time integral (FTI) is a simple multiplication of CF by time and, therefore, makes the assumption of a linear relationship between these two contributing factors. The EFFICAS I study demonstrated that minimum CF and FTI values are strong predictors of gap formation within a WACA segment; moreover, the investigators recommended a target CF of 20 g and a minimum FTI of 400 g∙s for each new lesion.19 The prospective EFFICAS II study showed a reduced rate of pulmonary vein reconnection (15% of pulmonary veins) with the use of an FTI target of 400 g∙s compared with not having CF available (28% of pulmonary veins) (TactiCath® Prospective Effectiveness Pilot Study [EFFICAS II]; ClinicalTrials.gov Identifier: NCT02131337).20
However, a significant limitation of FTI is that it does not take into account the power setting. The lesions depicted in Figure 1 were created with a constant CF of 10 g. Therefore, the top and bottom lesions (20 W for 30 s and 35 W for 30 s) both have an FTI value of 300 g∙s; however, there is a three-fold difference in lesion volume because of the different power settings used.15
Ablation Index
Ablation Index (AI) is a lesion quality metric incorporated into the CARTO® 3 (Biosense Webster Inc., Irvine, CA, USA) mapping system. AI differs from FTI by implementing a weighted logarithmic equation that includes power delivery, along with CF and time (Figure 2). Originally termed ‘force–power–time index’, it accurately predicts lesion depth in canine models.21,22

In clinical studies, AI predicted late WACA segment reconnection in a repeat left atrial mapping study 2 months after the initial PVI procedure. No reconnection was seen when the minimum AI value in a WACA segment was ≥480 for anterior/roof segments and ≥370 for posterior/inferior segments.23
The PRAISE study recruited 40 patients with persistent AF and prospectively used AI-guided PVI with target values of 550 for anterior/roof and 400 for posterior/inferior left atrial regions (Pulmonary Vein Reconnection Following Ablation Index-guided Ablation: a Success Evaluation [PRAISE]; ClinicalTrials.gov identifier: NCT02628730).24 Pulmonary vein reconnection was seen at repeat left atrial mapping procedures after 2 months in 22% of patients, affecting 7% of pulmonary veins.24
A further study compared patients with paroxysmal or persistent AF undergoing AI-guided PVI (using the same target values as in the PRAISE study), with propensity-matched patients receiving CF-guided PVI.25 AI-guided ablation was associated with significant reductions in acute pulmonary vein reconnection and arrhythmia recurrence. High rates of AF-freedom have been reported using AI-guided PVI in conjunction with tight control of ILD (CLOSE protocol) in a single-centre study.26 In the recent multicentre, prospective, non-randomized VISTAX study conducted at 17 European sites, PVI was guided by AI, with automated lesion tagging and ILD ≤6 mm (Evaluation of Ablation Index and VISITAG™ [ABI-173] [VISTAX]; ClinicalTrials.gov identifier: NCT03062046). The study found that this standardized paroxysmal AF ablation workflow aiming for contiguous lesions led to a rate of 12-month freedom from arrhythmias approaching 80%.27
Lesion Size Index
While AI considers CF, power and time, it does not provide information on lesion continuity, hence the need for tight control of ILD. Continuity is influenced by lesion dimensions, catheter movement between lesions and tissue depth at which it is assessed. Thus, lesion continuity requires uninterrupted ablated tissue extending between adjacent lesions from the endocardial to the epicardial surface.28
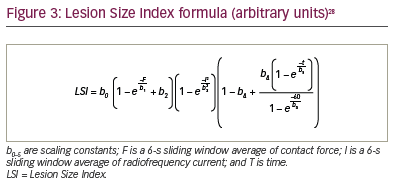
The Lesion Size Index (LSI) is a lesion quality metric available in the Ensite Precision™ (Abbott Laboratories, Chicago, IL, USA) electroanatomic mapping system. The formula incorporates a 6-second sliding window of average CF and RF current and, in a porcine recovery model, has indicated the degree of catheter movement permitted to maintain an uninterrupted line of transmural atrial ablation (Figure 3).28 An LSI value of 5 means 5 mm of catheter movement to the next lesion. Kanamori et al. have suggested LSI target values of 5.2 anteriorly and 4.0 posteriorly to avoid acute reconnection or dormant conduction.29
In 2018, the first prospective study of LSI compared 28 patients with paroxysmal AF undergoing LSI-guided PVI with 32 patients that had undergone non-CF-guided PVI. LSI-guided ablation produced improved clinical outcomes, with 89.3% AF-free survival at 17 months versus 65.6% in the non-CF-guided group, without an increase in complication rates.30
Impedance drop
Impedance drop during RF ablation is a marker of tissue heating and lesion formation. Generator impedance, which is a transthoracic measurement between the tip of the catheter and a patch placed on the patient’s skin, has been used for many years but is limited by a large ‘field of view’ and other factors, such as body habitus, fluid status and changes in lung volume.3
Conversely, local impedance (LI; measured by DirectSense™ [Boston Scientific, San Jose, CA, USA]) is a more focal impedance metric. Specifically, current injection from the distal electrode to the proximal electrode creates a local electric field. Distortion in that field as the catheter approaches tissue causes a change in the LI, and, when the electrical properties of tissue change with heating during RF application, a LI drop can be observed.31 In a preclinical study, LI value increased with greater catheter–tissue coupling independent of catheter orientation.31 Furthermore, LI drop predicted lesion depth, with higher starting (pre-ablation) LI resulting in a larger LI drop.
The LOCALIZE study showed that the magnitude of LI drop predicted both acute and chronic PVI in patients with paroxysmal AF (Electrical coupling information from the Rhythmia HDx system and DirectSense technology In subjects with paroxysmal atrial fibrillation [LOCALIZE]; ClinicalTrials.gov identifier: NCT03232645).4 For segments of the PVI ring where ILD was <6 mm, the optimal LI drops for chronic conduction block were found to be 16.9Ω for anterior/superior segments and 14.2Ω for posterior/inferior segments.4,32
Temperature
Tissue temperature is another output value that has, until recently, been difficult to use in clinical practice due to catheter tip irrigation impacting temperature measurements. However, two new catheters (QDot Micro™ [Biosense Webster Inc., Irvine, CA, USA] and DiamondTemp™ [Medtronic, Minneapolis, MN, USA]) are now available and are designed to allow temperature-controlled ablation. Both catheter designs have distally mounted thermocouples for a more accurate assessment of tissue temperature, with the DiamondTemp™ catheter incorporating industrial-grade diamond, a highly thermal-diffusive material, in the catheter tip for rapid tip cooling.5,33,34
The QDOT-FAST trial enrolled 52 patients and used the QDot Micro™ catheter to deliver a very high-power, short-duration (HPSD) ablation algorithm (90 W for 4 seconds) while maintaining the target temperature (Clinical Study for Safety and Acute Performance Evaluation of the THERMOCOOL SMARTTOUCH® SF-5D System Used With Fast Ablation Mode in Treatment of Patients With Paroxysmal Atrial Fibrillation [QDOT-FAST]; ClinicalTrials.gov Identifier: NCT03459196).5 This resulted in reduced procedure and RF ablation times, with 94.2% of patients remaining in sinus rhythm at 3 months. A larger study, Q-FFICIENCY (Evaluation of QDOT MICRO™ catheter for pulmonary vein isolation in subjects with paroxysmal atrial fibrillation [Q-FFICIENCY] ClinicalTrials.gov identifier: NCT03775512), involving 277 patients aims to demonstrate the safety and effectiveness of this catheter and is on-going.35
The first study to assess the DiamondTemp™ catheter was the TRAC-AF study (ACT DiamondTemp temperature-controlled and contact sensing RF ablation clinical trial for atrial fibrillation [RAC-AF]; ClinicalTrials.gov identifier: NCT02821351).33 In this study, 35 patients undergoing temperature-guided PVI with the DiamondTemp™ catheter had lower mean RF application and fluoroscopy times and reduced acute dormant reconnection compared with a control group undergoing CF-guided ablation. Twenty-three of the 35 patients who underwent temperature-guided PVI returned for a repeat left atrial mapping procedure after 3 months, with chronic pulmonary vein reconnection seen in only 26% of patients and 10% of pulmonary veins. Patients from the control group were not brought back for a repeat left atrial mapping study. A subsequent prospective multicentre trial (DiamondTemp™ Ablation System for the Treatment of Paroxysmal Atrial Fibrillation [DIAMOND-AF]; ClinicalTrials.gov identifier: NCT03334630) enrolling 482 patients proved non-inferiority in terms of clinical outcomes and lower total RF time, individual RF ablation duration and saline infusion for the DiamondTemp™ system compared with CF-sensing RF ablation in patients with paroxysmal AF.34
Both of these catheters are able to provide HPSD RF ablation by adjusting the power to keep tissue temperature at a certain level for a fixed time. By delivering shorter applications of higher power, there is a change in the shape of the resultant lesion. Enomoto et al. compared the lesion size from HPSD (50 W, 7 seconds) ablation with that from conventional power settings (25 W, 30 seconds) in an in vitro model.36 The authors concluded that, although the lesion volume was similar between groups, HPSD ablation created wider but shallower lesions compared with the control group (HPSD versus control lesion volume; mean ± SD: 29.6 ± 18.1 mm3 vs 35.5 ± 17.1 mm3, p=0.16; lesion diameter: 4.98 ± 0.91 mm vs 4.45 ± 0.74 mm, p=0.0095; lesion depth: 2.2 ± 0.76 mm vs 2.8 ± 1.56 mm, p=0.046).36
Discussion
To date, a number of RF lesion quality metrics have been developed to assist physicians in delivering durable, transmural, contiguous ablation lesions. Initial markers relied on input parameters, which were first evaluated in the TOCCATA study in 2011, which evaluated TactiCath™, the first available CF catheter.14 Subsequently, there was FTI, which was assessed in the EFFICAS I and II studies.19,20 While FTI represented a step forward from application duration or CF alone, it was limited by its failure to incorporate power delivery.15 AI and LSI, who incorporate power in a weighted formula with CF and time, now surpass FTI in common usage, with evidence of improved clinical outcomes.24,28
However, as AI and LSI incorporate CF, power and duration, they also retain the caveats of these factors. CF is not steady throughout the RF application and could be less accurate depending on the tip direction, especially in more parallel orientations.37 In addition, mapping catheters that are commonly used alongside the ablation catheter in the left atrium to assess for PVI can interact with the ablation catheter, potentially misleading the operator regarding the degree and direction of the applied force.38
While these metrics have significantly advanced the treatment of arrhythmias such as AF, a theoretical limitation is that they depend on input parameters with no direct assessment of tissue response to RF energy delivery. As a consequence, more recent lesion quality markers have focused on trying to measure tissue effect. LI provides a more refined version of generator impedance, which has been used for many years as a marker of tissue response to ablation, and predicts lesion depth and acute WACA segment reconnection.4,31 Measuring tissue temperature, which has long been of limited value in the era of irrigated catheters, is now possible due to novel catheter designs.36 The QDot Micro™ and DiamondTemp™ catheters both reduce RF times compared with CF-guided PVI.34,35 Attenuating the electrogram amplitude is another marker of tissue response to ablation and is widely used in clinical practice but is not currently incorporated in any of the composite lesion markers.39
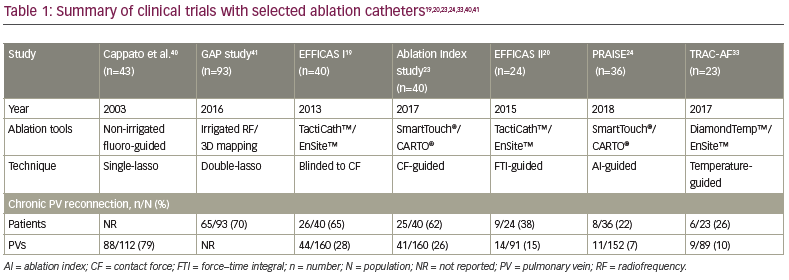
Direct, head-to-head, randomized controlled trials comparing these various lesion quality markers are currently lacking. In the absence of these, perhaps the ‘gold standard’ measure of the effectiveness of a lesion quality metric is the rate of pulmonary vein reconnection at a protocol-mandated repeat left atrial mapping procedure performed 2–3 months after PVI guided by that metric. This study design, with repeat procedures undertaken in all study participants irrespective of symptoms, provides an unbiased reflection of durable PVI and has been deployed in multiple studies of RF lesion quality markers, as well as other PVI techniques, such as the cryoballoon and laser balloon. Table 1 shows the evolution of ablation technology over the last 18 years, with accelerating developments in the past 8 years.19,20,23,24,33,40,41 These developments have translated into progressively lower rates of pulmonary vein reconnection at repeat left atrial studies. Nevertheless, guaranteed durable PVI following an initial RF ablation procedure has not yet been attained.24,33
Conclusion
To date, a number of RF lesion quality markers have been developed and evaluated. These include metrics that incorporate combinations of the input parameters of CF, time and power, including FTI, AI and LSI, and measures of output parameters reflective of tissue response to ablation, including LI drop and tissue temperature. These markers have led to significant improvements in RF delivery time, clinical outcomes and rates of chronic pulmonary vein reconnection, but durable PVI cannot yet be guaranteed.







