Introduction: Numerous imaging studies have firmly established epicardial adipose tissue (EAT) as an independent risk factor for all forms of AF. EAT hosts an array of immune cells and inflammatory mediators that can infiltrate the myocardium given the lack of fascial boundaries between them. However, the immune structure and cellular characterisation of EAT remains incompletely defined. This study sought to define the immunological signature of EAT critical to its role in the development of atrial fibrillation (AF) with a particular focus on
post-operative AF (POAF).
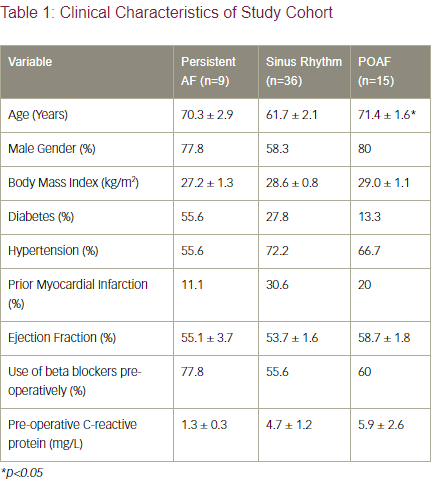
Methods: Adult patients with persistent AF and those with no prior history of AF undergoing cardiac surgery were recruited to undergo EAT, alongside subcutaneous adipose tissue (SAT) and pre-operative blood (adipose tissue and systemic controls respectively) sampling. Patients with coexisting systemic inflammatory disorders, those on immunomodulatory agents or patients with untreated thyroid dorders were excluded. Patient demographic and clinical characteristic data are summarised in Table 1. The blood and tissue samples were immediately taken to the laboratory for immune-cell isolation, antibody staining and flow cytometry analysis. Quantitative polymerase chain reaction (qPCR) was performed to assess inflammatory mediator expression in the blood and adipose tissue.
Results: 60 patients were recruited including a group of 9 patients with persistent AF. Of the 51 a priori sinus rhythm (SR) patients, 36 remained in SR post-operatively and 15 developed de novo POAF. No differences in absolute numbers of immune cells in the blood, EAT or SAT were detected between the three groups of patients. qPCR analysis of inflammatory mediator expression demonstrated POAF patients uniquely showed a 13-fold increase in the pro-fibrotic and inflammatory cytokine, interleukin-17 (IL-17, p<0.001) and 2.7-fold increase in chemokine ligand 17 (CCL17, p<0.05) expression exclusively within EAT.
Flow cytometry analysis demonstrated a significant increase in (p<0.005) in EAT-resident CD4+ memory T cell populations in persistent AF patients and a trend towards significance (p=0.14) in POAF patients.
Conclusions and Implications: Persistent AF and POAF patients demonstrate increased resident CD4+ memory T cell populations locally and exclusively in the EAT, suggesting the priming of antigen-specific CD4+ T cells must occur prior to the development of AF in patients who go on to develop de novo POAF. The elevated IL-17 levels seen in the EAT of POAF patients is consistent with animal models of fibrosis and AF, while CCL17 is known to induce chemotaxis of T cells to sites of inflammation.
Given POAF peaks at day 2, a rapid recall memory T cell response following the surgical insult would be consistent with the time course of POAF. Targeting these inflammatory mediators with monoclonal antibody approaches would provide a novel paradigm in the management of the inflammatory and fibrotic components of AF genesis.