Introduction: Atrial fibrillation (AF) is the most frequently encountered sustained arrhythmia. Electrical cardioversion is incompletely effective yet requires sedation and in-hospital monitoring. A “pill-in-the-pocket” Class IC anti-arrhythmic drug approach is often successful in restoring sinus rhythm but is limited by contraindications and safety concerns. This systematic review assessed the efficacy of single, high-dose oral amiodarone in converting AF within 48 hours of drug administration.
Methods: Studies were identified in MEDLINE and Embase without language restriction from database inception through May 2020. The proportion of patients converted to sinus rhythm after receiving amiodarone was extracted from all studies. Additionally, the risk ratio (RR) of successful cardioversion was extracted from placebo-controlled randomized controlled trials (RCTs). Weighted proportions and RRs were estimated using random effects meta-analysis techniques. A continuity correction was applied to studies with either zero or all events.
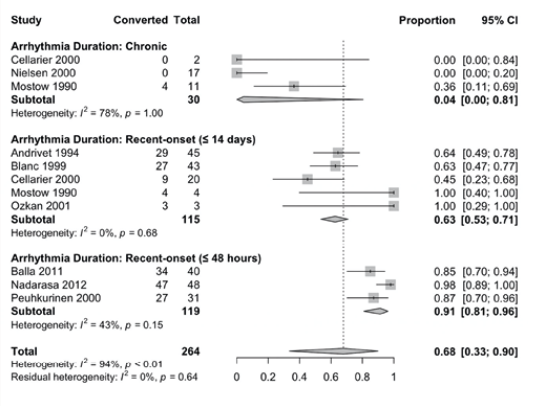
Results: Six single-arm observational studies (n = 150) and three clinical trials (n = 114) were included. Patients receiving amiodarone were 63 ± 6 years of age and 57% male. Successful cardioversion was achieved in 91% (95% confidence interval [CI]: 81% to 95%) of patients with acute AF (within 48 hours of onset), 63% (95% CI: 53% to 71%) of patients with recent-onset AF (within two weeks of onset), but only 4% (95% CI: 0% to 81%) of patients with long-standing persistent AF (at least one year). Of the three clinical trials, two were placebo-controlled, single-blind RCTs that compared amiodarone (n = 71) against matching placebo (n = 71) in patients within 48 hours of AF onset. Oral amiodarone significantly increased the likelihood of successful acute AF conversion relative to placebo (RR = 3.32, 95% CI: 1.67 to 6.61, p < 0.01).
Conclusions and Implications: A single, oral converting dose of amiodarone was largely effective in achieving sinus rhythm in patients with acute and recent-onset AF, but not long-standing persistent AF. The use of oral amiodarone as first-line therapy for recent-onset AF is appealing due to its convenience, cost-effectiveness, and acute safety profile. However, prior RCTs only included patients within 48 hours of AF onset. A placebo-controlled trial with expanded inclusion and adequate follow-up is warranted to determine the benefit of pill-in-the-pocket amiodarone for management of paroxysmal and acute persistent AF.
Figure: